noh lewis structure
NO Nitric Oxide Nitric oxide is a simple molecule which contains only two atoms. Also the above structure is more stable than the previous structures.

Ocl2 Lewis Structure Dichlorine Monoxide Molecules Lewis Electrons
For the NH2OH Lewis structure calculate the total number of valen.
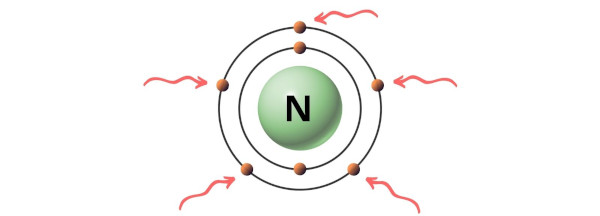
. Mark lone pairs Step 3. Nitrogen has five valence electrons in its outermost shell and Oxygen has six valence electrons. Following steps are required to draw the NO 2 lewis structure and they are explained in detail in this tutorial.
It is used to show how the electrons are arranged around individual atoms in a molecule. Compounds with unpaired electrons are sometimes referred to as free radicals This unpaired electron explains nitrogen dioxides reactive behavior as it has a strong desire to fill this open electron spot. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures.
Find total number of electrons of the valance shells of nitrogen and oxygen atoms Total electrons pairs Center atom selection Put lone pairs on atoms Check the stability and minimize charges on atoms by converting lone pairs to bonds. A simple method for writing Lewis Structures was given in a previous article entitled Lewis Structures and the Octet Rule. Therefore total valence electrons in NO 56 11 Step 3.
Put the least electronegative atom in the center. NO2- Lewis Structure is a complex structure because of the charges of atoms in NO2- ion. It should also have 6 lone pairs.
And in this case the most electronegative element is oxygen. A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitrosonium ionFor the NO structure use the periodic table to find the total number. The best Lewis structure is one that has the fewest formal charges the top structure.
This answer reserved by the author for Quora subscribers Access Guy Clentsmith s full answer archive. For Nitrogen Group 15 element number of valence. Therefore this structure is the most stable lewis structure of NO 3.
Its lewis structure can be drawn by following VSEPR rule. As nitrogen is the least. Number of electrons in the valence shells are used to draw the stable lewis structure and it is used find the resonance structures of NO.
The Lewis structure proposed by Gilbert Newton Lewis who introduced it for the first time in 1916 is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species. What is the Lewis structure of NO2. H 2 S NCl 3 OH -.
According to the Lewis structure of NO3 molecule here central atom is nitrogen which is bonded with three oxygen atoms and it contains zero lone pairs. In highly concentrated amounts it is toxic. Mark charges Step 4.
The Lewis structure of nitrogen dioxide is also interesting because there is a single unpaired valence electron on the central nitrogen atom. Around 120 Å is the bond length. NO Nitric oxide is an oxide of nitrogen.
NO lewis structure One lone pair over N atom and two lone pairs over O atom. View the full answer. Minimize charges Step 5.
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. Steric number 30 3. Electrons are shown as dots or for bonding electrons as a line between the two atoms.
Well you got 2 6 12 electrons from the oxygen and 1 5 5 O. NOCl CF 2 Cl 2 HCN. Nitric oxide is an important signaling molecule in vertebrate organisms and is one of the main components of acid rain.
Lewis structures are also known as. It is the simplest of the nitric oxides compounds containing nitrogen and oxygen and has a molar mass of 301 gmol. Minimize charges again if there are Lets break down each step in detail.
Here you can see the Lewis Structure for NO2- below. Include all lone pairs of. Part E NOH Draw the Lewis dot structure for NOH3.
Draw sketch Step 2. Create your account View this answer We are asked to draw the Lewis structure for NO N O. When we examine the nitrate ion NO2- we notice that it contains two bond pairs one lone pair of electrons.
The Lewis Structures are the diagrammatic representation of bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. CH 4 NH 3 I 2. Now let us draw these electrons as.
Nitride ion or NO2- has -1 charge on 1 oxygen atom. This is the best answer based on feedback and ratings. As a result NO2 has a bond angle of around 134 degrees.
One N atom and one O atom. Nitric oxide NO is a gaseous compound composed out of a single nitrogen atom and a single oxygen atom. Note the presence of the -1.
Hence steric number of central nitrogen atom in NO3 molecule is three which shows sp2 hybridization. Thus the Lewis structure of NO is Lewis Dot Structure of NO Nitrogen Monoxide Watch on Answer link. There is a single electron on the N atom as N contains five electrons in the valence shell in order to gain stability the free-electron over N forms a bond with O and there will be a partial triple character observed in.
There are TWO regions of electron density around the central nitrogenmath_ O-N. Lewis structures can be used to represent valence shell electrons in a chemical bond. NO or nitric oxide has two atoms.
H always goes outside. Put two electrons between atoms to form a chemical bond. N O.
NOH3 is actually NH2OH. Find the total valence electrons for the molecule. Steps for Writing Lewis Structures.
This is okay because the structure with a negative charge on the most electronegative atom is the best lewis structure. Now counting the number of valence electrons in the molecule. A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitric acidFor the NO structure use the periodic table to find the total number of va.
To draw the Lewis structure of NOF we first need to choose a central atom. Nitrogen atom forms a lone bond with one of the oxygen atoms and it forms a double bond with other oxygen atom. Heres how you can draw the NO lewis structure step by step.
There are two bonded pairs and an unpaired electron in the Lewis structure for NO2.
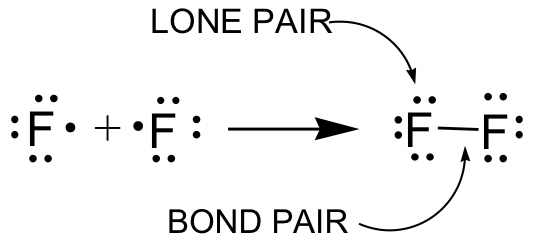
1 2 Lewis Structure Organic Chemistry I

Ch3oh Lewis Structure Methanol
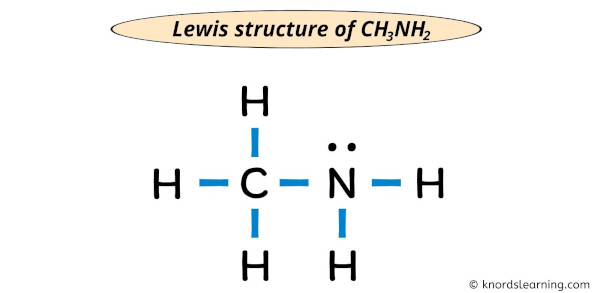
Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw

7 3 Lewis Symbols And Structures Chemistry

Chf3 Lewis Structure Fluoroform Happy Birthday Quotes For Friends How To Find Out Happy Birthday Quotes

Hcn Lewis Structure Hydrogen Cyanide Molecules Lewis Biology

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube
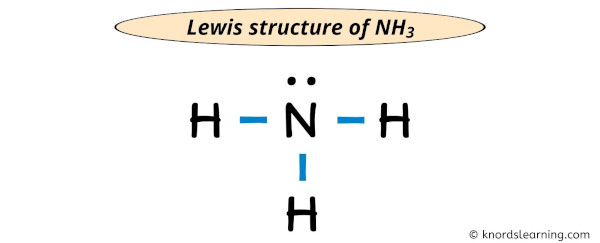
Lewis Structure Of Nh3 Ammonia With 6 Simple Steps

Lewis Dot Structure For Na2o Sodium Oxide Ionic Bonding Ionic Compound Ap Chemistry
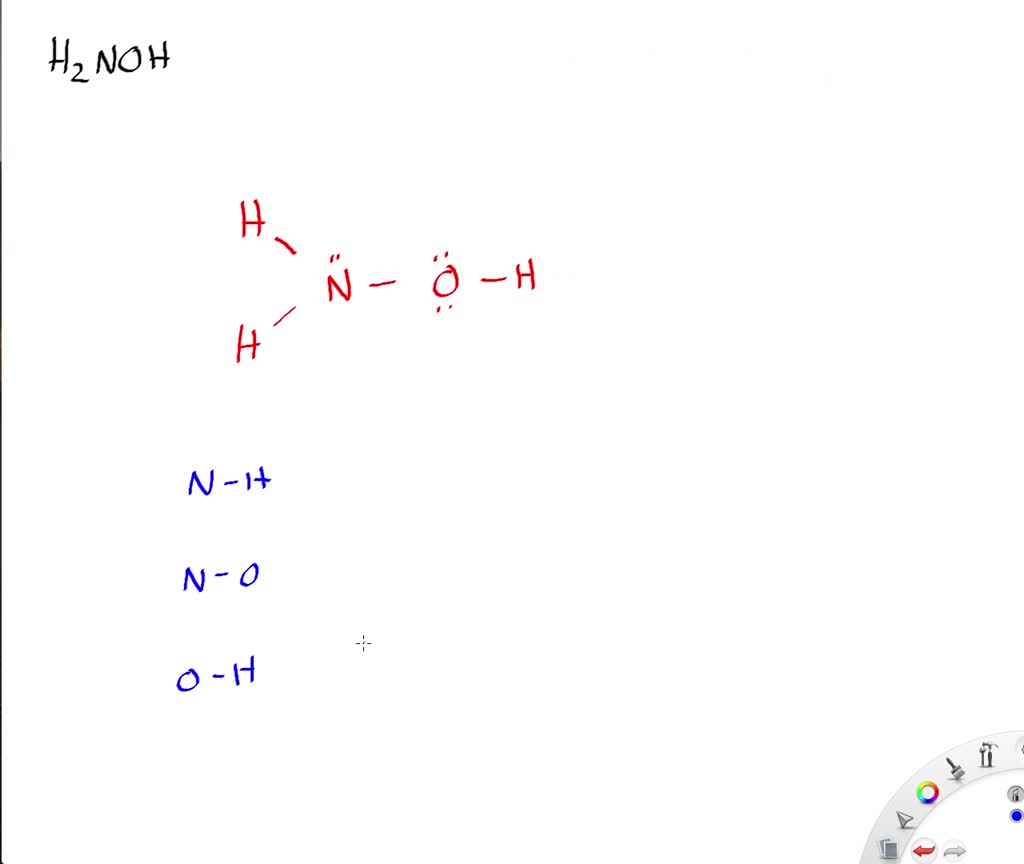
Solved Write A Lewis Structure Of The Hydroxylamine Molecule Mathrm H 2 Noh Then With Data From Table 10 2 Determine All The Bond Lengths
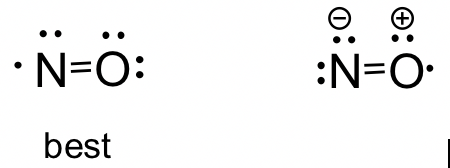
1 2 Lewis Structure Organic Chemistry I
How Is The Lewis Dot Structure For Hno3 Determined Quora

Lewis Structure Of Nh3 Ammonia With 6 Simple Steps

Nh2oh Lewis Structure How To Draw The Lewis Structure For Nh2oh Hydroxylamine Youtube
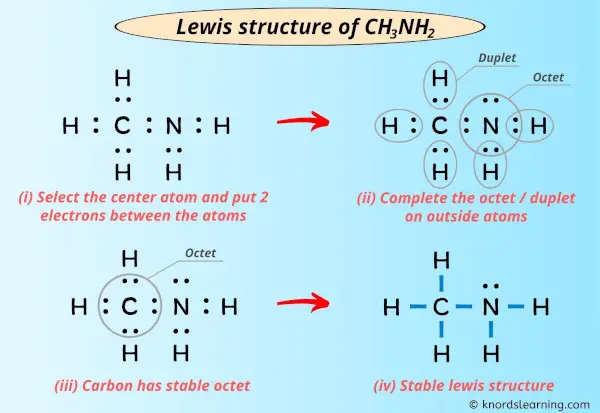
Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw

Nh2oh Lewis Structure Hydroxylamine Math Lewis Chemical Formula

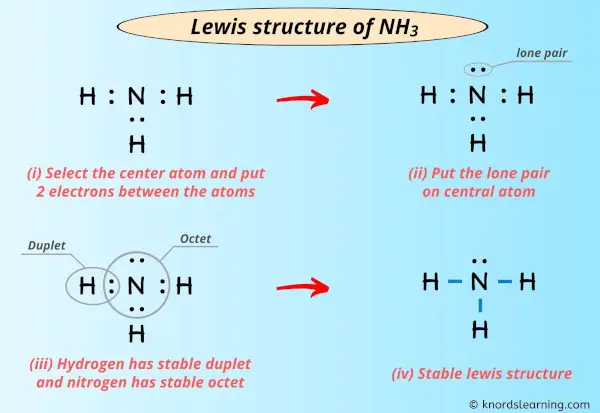
Comments
Post a Comment